Abstract
This research delves into the cognitive effects of HIV infection (HIV) and chronic alcoholism (ALC), both independently and when co-occurring (HIV+ALC). Understanding these conditions is critical, especially given the prevalence of comorbidity and the potential for compounded cognitive impairments. Furthermore, as individuals with HIV live longer due to advancements in treatment, age and disease-related factors become increasingly important in understanding cognitive health. This study investigates the specific patterns of executive function and episodic memory deficits associated with HIV, ALC, and their comorbidity, providing insights into the neurological impact of these conditions. This analysis is crucial for understanding conditions potentially related to memory#0001 bruce park and broader cognitive health.
Methods
To assess cognitive function, we administered a comprehensive battery of both traditional and computerized neuropsychological tests to four groups: individuals with HIV, individuals with ALC, individuals with comorbid HIV and ALC (HIV+ALC), and a group of normal controls (NC). Test scores were adjusted for age and education using Z-scores. We calculated composite scores for Executive Function and Episodic Memory by averaging scores from selected tests. For tests measuring both accuracy and response time, we also calculated efficiency scores to evaluate the balance between speed and precision.
Results
Our findings revealed significant cognitive differences between the groups. The HIV, ALC, and HIV+ALC groups all exhibited lower scores on both the Executive Function and Episodic Memory Composite scores compared to the NC group. Notably, the HIV+ALC group showed even greater impairment in Episodic Memory compared to both the ALC and HIV groups alone.
Examining specific cognitive domains, we observed distinct patterns of impairment. The ALC group demonstrated difficulties in planning and free recall of visuospatial information. In contrast, the HIV group showed more pronounced impairments in psychomotor speed, sequencing abilities, narrative free recall, and pattern recognition. Across all three clinical groups (HIV, ALC, and HIV+ALC), we found lower decision-making efficiency scores compared to the NC group, indicating a general deficit in this area.
Furthermore, we investigated the role of age and alcohol consumption in cognitive performance within the clinical groups. In the ALC group, both age and lifetime alcohol consumption were identified as independent predictors of both Executive Function and Episodic Memory Composite scores. However, in the HIV+ALC group, age emerged as the sole unique predictor of Episodic Memory Composite score, suggesting a potentially different interplay of factors in comorbid conditions.
Conclusions
This study highlights the distinct and overlapping patterns of cognitive impairment associated with HIV infection, chronic alcoholism, and their comorbidity. These findings have significant implications for understanding the specific brain systems affected by each condition. The compounded cognitive deficits observed in the HIV+ALC group underscore the complexities of dual diagnoses and the potential for exacerbated cognitive decline, particularly with aging. These results emphasize the need for tailored clinical approaches to address the unique cognitive challenges faced by individuals with HIV, alcoholism, and especially those with both conditions.
Keywords: HIV Infection, Alcoholism, Executive Functions, Episodic Memory, Comorbidity
Chronic alcoholism (ALC) frequently co-occurs with HIV infection (HIV) [Conigliaro et al., 2006; Gongvatana et al., 2014], with rates in HIV-infected individuals being approximately twice that of the general population [Justice et al., 2010]. Both conditions are independently associated with specific profiles of cognitive impairment, particularly affecting executive functions [ALC: [Beatty et al., 1993; Bernardin et al., 2014; Nixon and Parsons, 1991; Noel et al., 2001; Oscar-Berman and Marinkovic, 2007]; HIV: [Giesbrecht et al., 2014; Heaton et al., 2011; Woods et al., 2009]] and episodic memory [ALC: [Becker et al., 1983; Parsons, 1977; Pitel et al., 2007; Riege et al., 1981; Sullivan et al., 2000]; HIV: [Maki et al., 2015; Stout et al., 1995]].
Despite the advent of combination and highly-active antiretroviral therapy (CART/HAART), neurocognitive impairment remains a significant concern in HIV infection [Clifford and Ances, 2013; Heaton et al., 2011; Saylor et al., 2016]. However, the focus of HIV-related cognitive research has shifted from primarily motor-related deficits, such as slowed motor speed and information processing [Becker et al., 1997], to impairments in higher-order cognitive domains, specifically executive functions and episodic memory [Heaton et al., 2011; Sacktor and Robertson, 2014]. Executive functions, encompassing planning, efficiency, monitoring, and strategic memory processes, are particularly vulnerable in HIV infection [Cattie et al., 2012; Woods et al., 2013]. With antiretroviral treatments extending the lifespan of individuals with HIV, it is now crucial to understand how other factors, such as aging, medication neurotoxicity, chronic inflammation, co-occurring medical conditions (e.g., Hepatitis C), psychiatric comorbidities (e.g., depression), and substance use disorders [Hardy and Vance, 2009; Sheppard et al., 2015; Woods et al., 2009], can influence and modify cognitive deficits in this population.
Cognitive impairments associated with chronic alcoholism share overlaps with those seen in HIV infection, particularly in executive functions and episodic memory [Fama et al., 2004; Glenn and Parsons, 1992; Oscar-Berman et al., 2004; Tivis et al., 1995]. Working memory, cognitive flexibility, inhibition, processing speed, concept formation, planning, and problem-solving are among the executive function components most susceptible to impairment in chronic alcoholism [Bernardin et al., 2014]. Studies have shown deficits in attentional set-shifting and working memory in abstinent alcohol-dependent individuals, with the most pronounced impairments observed in those abstinent for a year or less [Kopera et al., 2012]. Episodic memory deficits in chronic alcoholism include impairments in encoding, retrieval, and the processing of spatiotemporal context [Pitel et al., 2007]. Efficiency scores, reflecting the delicate balance between speed and accuracy in tasks involving executive functions and episodic memory, are also compromised in alcoholism [Nixon and Bowlby, 1996; Nixon and Parsons, 1991]. Factors such as age, drinking history (duration and patterns), and comorbid medical or psychiatric conditions can further influence cognitive deficits in chronic alcoholism.
Individuals facing both HIV and alcoholism (HIV+ALC) are at an elevated risk for compounded cognitive challenges, impacting verbal reasoning, working memory, and episodic memory [Fama et al., 2009; Fama et al., 2011; Gongvatana et al., 2014; Green et al., 2004; Rothlind et al., 2005]. Even a past history of alcoholism can negatively affect cognitive function in HIV-infected individuals, especially in working memory, episodic memory, and visuomotor skills [Gongvatana et al., 2014]. These compounded deficits may arise from the combined effects of dysfunction in distinct neural systems [e.g., frontocerebellar circuits in ALC [Oscar-Berman and Marinkovic, 2007; Sullivan et al., 2003] and frontostriatal circuits in HIV [Ipser et al., 2015]] and overlapping neural systems [e.g., the limbic system (ALC: Sullivan et al., 1995 HIV: Ortega et al., 2013)]. However, the specific patterns and severity of executive function and episodic memory deficits in ALC, HIV, and HIV+ALC are variable, both across and within these conditions, and cognitive deficits are not universally present [ALC: (Arciniegas and Beresford, 2001; Fein and Landman, 2005) ; HIV: [Woods et al., 2009] ].
This study aimed to investigate the component processes of executive function and episodic memory in individuals with alcoholism, HIV infection, and their comorbidity, and to explore the relationships between these cognitive processes and demographic and disease-related variables. We utilized traditional and computerized neuropsychological tests known for their sensitivity in assessing these cognitive domains in alcoholism and HIV infection. We tested two primary hypotheses: (1) Individuals with ALC and HIV would show impairments in component processes of executive functions and episodic memory compared to healthy controls, and the comorbid group (HIV+ALC) would exhibit greater cognitive compromise than either single-diagnosis group. We also hypothesized that different executive function components would be more affected in ALC (decision making, complex attention, set shifting) versus HIV (psychomotor speed, planning, monitoring, sequencing) due to distinct neural pathways involved (frontocerebellar in ALC and frontostriatal in HIV). We anticipated that shared limbic system involvement would lead to similar episodic memory component processes being affected in both HIV and ALC. (2) The level of executive function and memory impairment in the clinical groups would be associated with specific demographic and disease-related variables. Specifically, we predicted that older age (beyond normal aging effects) and higher lifetime alcohol consumption would correlate with greater cognitive deficits in the alcohol groups, and lower CD4 cell counts would be associated with greater cognitive deficits in the HIV infection groups.
Materials and Methods
Participants
The study included 36 HIV-seropositive individuals (HIV), 39 HIV-seronegative individuals meeting DSM-IV criteria for alcohol dependence within 3 years prior to study entry (ALC), 42 individuals seropositive for HIV and meeting criteria for alcohol dependence within the same timeframe (HIV+ALC), and 31 individuals with neither HIV nor alcohol dependence or abuse (NC). Participants were drawn from an ongoing longitudinal study examining the effects of HIV and alcohol on brain structure and function. All participants provided written informed consent, and the study was approved by the Institutional Review Boards of SRI International, Stanford University, and Santa Clara Valley Medical Center.
At the time of testing, 20 HIV and 24 HIV+ALC participants met CDC criteria for AIDS (CD4 cell count <200 cells/mm3). In the ALC group, 56.4% had a history of non-alcohol substance abuse or dependence, with a median sobriety duration of 115 weeks. In the HIV groups, 44.4% of HIV-only and 87.2% of HIV+ALC participants had a history of non-alcohol substance abuse or dependence, with median sobriety durations of 724 weeks and 121 weeks, respectively. Cocaine and cannabis were the most commonly reported drugs of abuse. At the time of testing, cannabis abuse criteria were met by 3 participants (1 HIV and 2 HIV+ALC) within the past month. ALC participants had a mean alcohol dependence remission time of 87 weeks, and HIV+ALC participants had a mean of 211 weeks. The median age of alcohol dependence onset was approximately 20-21 years across the ALC and HIV+ALC groups. Lifetime and current nicotine use varied significantly across the groups (x2(3)=26.99, p<0.001).
The HIV, ALC, and HIV+ALC groups were significantly older than the NC group and had fewer years of formal education and lower NART-IQ scores (estimated IQ) (Table 1). Socioeconomic status (SES), as assessed by education and occupation level [Hollingshead, 1975], was lower in the clinical groups compared to controls. As expected, the ALC and HIV+ALC groups reported significantly higher lifetime alcohol consumption than the HIV and NC groups, with the ALC group reporting the highest consumption. Depressive symptom severity, as measured by the Beck Depression Inventory-II, was higher in all three clinical groups compared to the control group. CD4 cell counts were comparable between the HIV and HIV+ALC groups. Total scores on the Dementia Rating Scale – 2 [Mattis, 2004], assessing overall cognitive function, were lower in the HIV and HIV+ALC groups compared to controls, and lowest in the HIV+ALC group compared to the ALC group.
Table 1. Demographic characteristics of subjects (mean, standard deviation, range)
| Group | Age (yrs) | Education (yrs) | SES* | NART IQ† | Lifetime AlcoholConsumption (kg) | Beck DepressionInventory-II | CD4 count | DementiaRating Scale |
|---|---|---|---|---|---|---|---|---|
| HIV-infection (HIV n=36) | 49.5 (8.8) 25 to 64 | 13.4 (2.8) 9 to 19 | 40.2 (14.9) 11 to 58 | 105.1 (9.2) 90 to 123 | 69.5 (64.1) 1 to 289 | 11.1 (9.7) 0 to 32 | 576.5 (270.0) 88 to 1372 | 136.5 (5.2) 121 to 144 |
| Alcoholism (ALC n=39) | 48.4 (9.7) 26 to 66 | 13.5 (2.3) 10 to 21 | 41.5 (12.9) 18 to 65 | 106.7 (9.5) 90 to 124 | 1257.1 (918.6) 227 to 4438 | 7.7 (5.7) 0 to 22 | NA | 138.5 (4.6) 127 to 144 |
| HIV-infection with Alcoholism (HIV+ALC n=39) | 50.1 (6.8) 31 to 64 | 13.0 (2.2) 8 to 18 | 43.8 (11.5) 11 to 62 | 105.6 (8.3) 91 to 125 | 843.2 (607.6) 22 to 2504 | 11.0 (9.0) 0 to 36 | 551.5 (325.5) 61 to 1179 | 135.8 (4.5) 127 to 143 |
| Control (NC n=31) | 44.1 (9.8) 25 to 62 | 15.1 (1.9) 11 to 18 | 30.7 (11.3) 11 to 58 | 112.5 (9.3) 92 to 126 | 23.9 (35.1) 0 to 163 | 3.0 (5.3) 0 to 12 | NA | 139.9 (2.6) 135 to 144 |
| Follow-up t-tests | p=.031 HIV,ALC,HIV+ALC>NC | p=.002 HIV,ALC, HIV+ALC < NC | p=.0003 HIV,ALC, HIV+ALC>NC | p=.006 HIV,ALC, HIV+ALC < NC | p=.0001 ALC,HIV+ALC>HIV, NC ALC>HIV+ALC | p < .0001 HIV,ALC, HIV+ALC>NC | ns | p=.0007 HIV,HIV+ALC<NC HIV+ALC<ALC |
*SES – Socioeconomic Status based on education and occupation
†NART – National Adult Reading Test – estimated IQ
NA – Not applicable
Procedure
Participants underwent screening using the Structured Clinical Interview for DSM-IV (SCID) [First et al., 1998] and structured health questionnaires. Exclusion criteria included less than 8 years of education, significant medical history (e.g., epilepsy, stroke, multiple sclerosis, uncontrolled diabetes, loss of consciousness > 30 minutes), psychiatric disorders (schizophrenia, bipolar I disorder), neurological disorders (neurodegenerative disease) other than those directly related to their clinical group. Additional exclusion criteria were recent substance dependence (excluding alcohol in clinical groups), serious medical conditions or HIV-related opportunistic infections in the HIV and HIV+ALC groups, and any DSM-IV Axis I disorder in the NC group.
All participants completed a semi-structured interview [Skinner, 1982; Skinner and Sheu, 1982] to quantify lifetime alcohol consumption. Depressive symptoms were assessed using the Beck Depression Inventory-II [Beck et al., 1996]. Blood samples were collected to determine HIV status, plasma viral load, and CD4 cell count. At recruitment, all HIV-positive individuals had CD4 counts >100 cells per mm3 and a Karnofsky score >80, indicating adequate functional abilities [Karnofsky, 1949].
Executive functions (working memory, planning, problem-solving, decision-making) and explicit memory (immediate free recall, recognition) were assessed using traditional and computerized tests. The Trail Making Test Part B [Borkowski et al., 1967; Reitan, 1958] was used to assess working memory and planning by requiring participants to sequence numbers and letters alternately and in ascending order. The Logical Memory subtest of the Wechsler Memory Scale – Revised (WMS-R) (Wechsler, 1987) assessed immediate verbal memory by requiring recall of two short narratives. The Rey-Osterrieth Complex Figure (ROCF) [Osterrieth and Rey, 1944; Rey, 1942] assessed immediate visual memory by requiring participants to draw a complex figure from memory after copying it. All traditional tests were administered and scored according to standardized protocols.
Computerized measures were administered using subtests from the Cambridge Neuropsychological Test Automated Battery (CANTAB) [Morris et al., 1987; Sahakian and Owen, 1992], which measures accuracy and response time for various executive function and episodic memory components. Seven CANTAB subtests were used:
- Motor Screening (MOT): Assesses basic sensorimotor function and task comprehension. Participants touch flashing crosses on the screen to ensure understanding of instructions and touchscreen navigation.
- Intra-Extra Dimensional Set Shift (IED): Evaluates rule acquisition and reversal, sensitive to frontostriatal dysfunction [Heaton, 1981]. The dependent measure is the number of extra-dimensional errors.
- Stockings of Cambridge (SOC): Assesses planning and working memory. Participants solve increasingly complex spatial problems by moving colored balls on pegs to match a target configuration. The dependent measure is the number of problems solved in the minimum number of moves.
- Cambridge Gambling Task (CGT): Measures decision-making and risk-taking. Participants bet on whether a target is behind blue or yellow squares, with odds clearly displayed. Accuracy reflects decision quality based on betting on the color with better odds. Response time is the average deliberation time for bet selection.
- Spatial Working Memory (SWM): Assesses the ability to maintain and manipulate spatial information. Participants find a hidden blue token in boxes, and errors are recorded for returning to already checked boxes or boxes previously containing a token. The dependent measure is the number of between-trial errors.
- Spatial Recognition Memory (SRM): Assesses short-term spatial memory. Participants view a white square in five different locations and then identify the previously presented locations from two choices. Dependent measures are the number correct and mean response latency for correct items.
- Pattern Recognition Memory (PRM): Assesses visual pattern recognition. Participants memorize patterns and then select the seen pattern in a two-choice forced recognition test. Dependent measures are the number correct and mean response latency for correct items.
Examples of the IED, SOC, and CGT subtests are available in supplemental materials.
Statistical Analyses
Conceptually-derived composite scores were calculated for executive function and episodic memory (Table 2). Age- and education-corrected Z-scores were calculated for individual test parameters based on the normal control group (NC mean ± standard deviation: Z=0±1) for direct comparisons. Scores where higher raw scores indicated worse performance were multiplied by -1, so lower Z-scores consistently indicated poorer performance. Efficiency ratio scores (accuracy/response time) were calculated for tests with both accuracy and response time measures (CGT, SRM, PRM).
Table 2. Executive Function and Episodic Memory Composite Scores
| Executive Function Composite |
|---|
| Spatial Working Memory (CANTAB) – Number of between trial errors |
| Trails B – Time to completion |
| Cambridge Gambling Test (CANTAB) – Quality of decision making on ascending trials |
| Stockings of Cambridge (CANTAB) – Number of problems solved in minimal moves |
| Intra-Extra Dimensional Set Shift (CANTAB) – Number of extradimensional errors |
| Episodic Memory Composite |
| Logical Memory (WMS-R) – Number of details recalled on immediate memory trial |
| Rey-Osterrieth Complex Figure – Number of details recalled on immediate memory trial |
| Spatial Recognition (CANTAB) – Number Correct |
| Pattern Recognition (CANTAB) – Number Correct |
Between-group differences were analyzed using ANOVAs. Bonferroni correction was applied for two-group post-hoc analyses when omnibus group differences were significant to control for Type I error. Nonparametric analyses (Mann-Whitney U test) were used to assess within-group differences (e.g., cognitive impairment in individuals with Hepatitis C or AIDS vs. those without). Pearson product-moment correlations were used to test relationships between demographic and disease-related variables and composite scores, with Bonferroni correction applied for family-wise comparisons (p < 0.025). Multiple regression analyses were used to examine the unique contributions of age and lifetime alcohol consumption to cognitive composite scores.
Results
Between Group Analyses
Groups did not differ significantly on the CANTAB motor screening subtest (MOT: F(3,141)=1.55, p=.20), indicating comparable sensorimotor accuracy and ability to understand computerized task demands.
Executive Function Composite Score
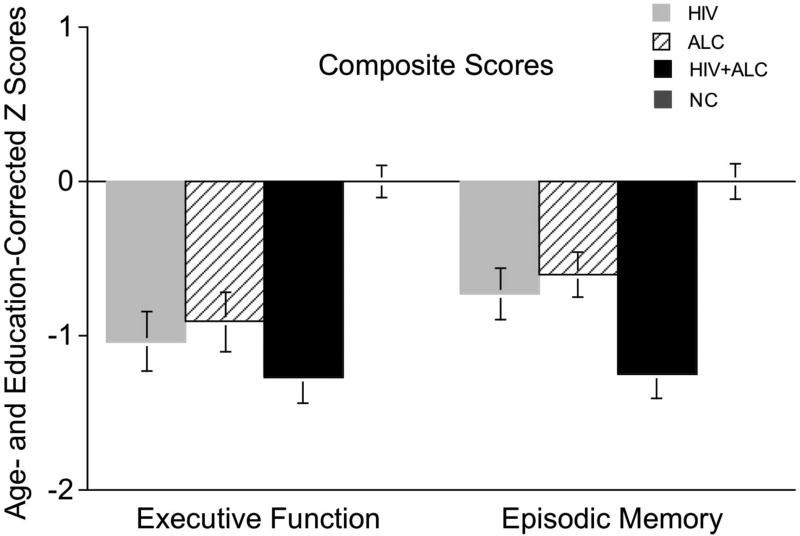
Significant group differences were found on the Executive Function Composite score [F(3,141)=9.27, p<0.0001] (Figure 1). Post-hoc analyses revealed that all three clinical groups (HIV+ALC, HIV, ALC) scored significantly lower than the NC group (HIV+ALC vs. NC p<0.0001; HIV vs. NC p<0.0001; ALC vs. NC p=0.0004).
Figure 1. Age- and education-corrected Z scores for Executive Function and Episodic Memory Composites across groups.
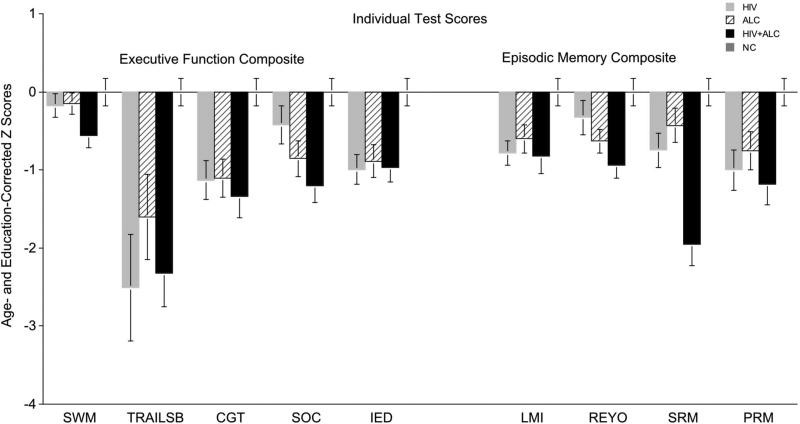
Examining individual tests within the Executive Function Composite, significant group differences were observed on Trails B [F(3,137)=4.63, p=.004], Cambridge Gambling Test [F(3,141)=5.38, p=.002], Stockings of Cambridge [F(3,141)=5.23, p=.002], Intra-Extra Dimensional Set Shift Test [F(3,141)=5.58, p=.001], and a trend was noted for Spatial Working Memory [F(3,141)=2.30, p=.08] (Figure 2). Raw scores for these tests are in Table 3, and significant two-group comparisons are in Table 4.
Figure 2. Age- and education-corrected Z scores for individual tests within Executive Function and Episodic Memory Composites. SWM=Spatial Working Memory; TRAILSB=Trail Making Part B; CGT=Cambridge Gambling Test; SOC=Stockings of Cambridge; IED=Intra-Extra Dimensional Set Shift; LMI=Logical Memory I; REYO=Rey-Osterrieth Complex Figure; SRM=Spatial Recognition Memory; PRM=Pattern Recognition Memory
Table 3. Raw Test Scores for Variables in Executive Function and Episodic Memory Composites (mean, standard deviation, range)
| HIV | ALC | HIV+ALC | NC | |
|---|---|---|---|---|
| Executive Function Composite | ||||
| Spatial Working Memory (Between trial errors) | 32.03 (19.44) 0 to 89 | 31.28 (18.94) 1 to 75 | 41.56 (20.51) 4 to 77 | 23.13 (21.94) 0 to 70 |
| Trails B (Time to complete in seconds) | 103.06 (70.66) 30 to 371 | 87.89 (56.10) 31 to 292 | 100.38 (44.19) 47 to 240 | 59.39 (17.62) 29 to 105 |
| Cambridge Gambling Test (Quality of Decision Making) | 0.78 (0.18) .38 to 1 | 0.77 (0.19) .46 to 1 | 0.75 (0.20) .38 to 1 | 0.91 (0.14) .41 to 1 |
| Stockings of Cambridge (Number of problems completed in minimal moves) | 7.97 (2.27) 5 to 12 | 7.38 (2.17) 3 to 12 | 6.74 (2.12) 4 to 11 | 9.00 (1.59) 4 to 12 |
| Intra – Extra Dimensional Set Shift (Number of extradimensional errors) | 15.28 (9.59) 1 to 28 | 14.72 (10.94) 1 to 33 | 14.72 (10.70) 0 to 30 | 8.77 (9.62) 0 to 33 |
| Episodic Memory Composite | ||||
| Logical Memory (Number of details correct on Immediate Recall) | 21.72 7.43 10 to 36 | 22.86 7.87 11 to 37 | 21.11 8.02 3 to 34 | 27.97 7.66 11 to 42 |
| Rey-Osterrieth Complex Figure (Number of details correct on Immediate Recall) | 14.01 (7.12) 2.5 to 30.5 | 12.31 (5.74) 0 to 29 | 10.10 (5.81) 0.5 to 30.5 | 17.53 (5.84) 8 to 32 |
| Spatial Recognition (Number Correct) | 16.11 (2.49) 10 to 20 | 16.64 (2.40) 10 to 19 | 13.92 (3.09) 1 to 18 | 17.32 (1.83) 14 to 20 |
| Pattern Recognition (Number Correct) | 20.42 (2.93) 12 to 24 | 20.87 (2.82) 13 to 24 | 20.03 (3.09) 11 to 24 | 22.42 (1.57) 18 to 24 |
Table 4. Significant two-group comparisons (Bonferroni corrected p < 0.0083)
| Executive Function Composite | p value |
|---|---|
| Trails B | HIV vs. NC |
| HIV+ALC vs. NC | |
| Cambridge Gambling Test | ALC vs. NC |
| HIV vs. NC | |
| HIV+ALC vs. NC | |
| Stockings of Cambridge | ALC vs. NC |
| HIV+ALC vs. NC | |
| HIV+ALC vs. HIV | |
| Intra – Extra Dimensional Set Shift | ALC vs. NC |
| HIV vs. NC | |
| HIV+ALC vs. NC | |
| Episodic Memory Composite | |
| Logical Memory | HIV vs. NC |
| HIV+ALC vs. NC | |
| Rey-Osterrieth Complex Figure | ALC vs. NC |
| HIV+ALC vs. NC | |
| Spatial Recognition | HIV+ALC vs. NC |
| HIV+ALC vs. ALC | |
| HIV+ALC vs. HIV | |
| Pattern Recognition | HIV vs. NC |
| HIV+ALC vs. NC |
Episodic Memory Composite Score
Significant group differences were also observed for the Episodic Memory Composite score [F(3,141)=11.31, p<0.0001] (Figure 1). Similar to executive function, all three clinical groups scored lower than NC (HIV+ALC vs. NC p<0.0001; HIV vs. NC p<0.0001; ALC vs. NC p=0.0002), with the HIV+ALC group scoring lower than both the HIV (p=0.0004) and ALC (p=0.0003) groups. At the individual test level, group differences were significant across all memory measures: WMS-R Logical Memory Recall [F(3,136)=3.76, p=.012], Rey-Osterrieth Complex Figure Recall [F(3,140)=4.76, p=.003], Spatial Recognition Memory [F(3,141)=13.23, p<0.0001], and Pattern Recognition Memory [F(3,141)=4.18, p=.007]. Raw scores are in Table 3, and two-group comparisons are in Table 4.
Efficiency Scores
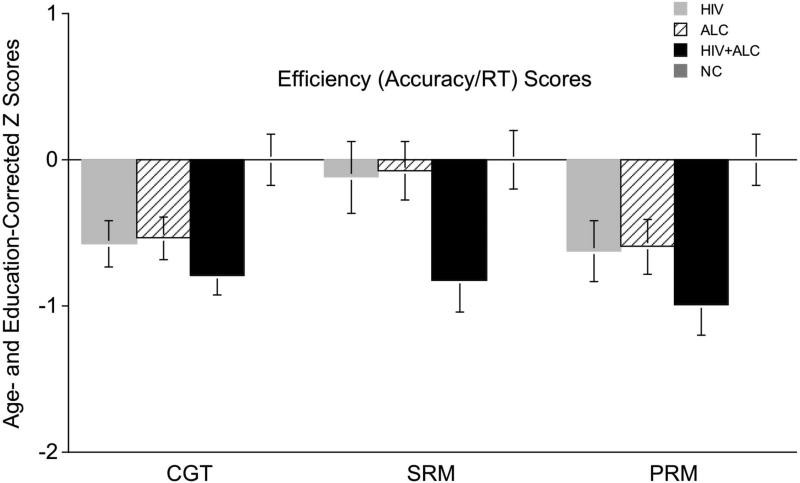
Efficiency ratios (accuracy/response time) were calculated for tests with response time scores related to accuracy. Response times are in Table 5. Efficiency ratios, corrected for age and education, showed significant group differences for all measures (CGT: F(3,141)=4.56, p=.004; SRM: F(3,141)=3.39, p=.02; PRM: F(3,141)=4.10, p=.008) (Figure 3).
Table 5. Raw Test Scores for Response Times for efficiency ratios (mean, standard deviation, range)
| HIV | ALC | HIV+ALC | NC | |
|---|---|---|---|---|
| Cambridge Gambling Test (Deliberation Time, ms) | 3193.0 (1133.7) 1236 to 6677 | 3204.2 (1234.6) 1198 to 7976 | 3693.4 (1804.0) 1220 to 11004 | 2732.7 (1224.2) 1310 to 6722 |
| Spatial Recognition Memory (Mean Latency Time, ms) | 2226.8 (612.3) 1009 to 3946 | 2267.8 (731.1) 1325 to 3946 | 2407.4 (720.3) 1162 to 4902 | 2071.5 (418.9) 1302 to 3084 |
| Pattern Recogniton Memory (Mean Latency Time, ms) | 1964.5 (466.6) 898 to 3165 | 2008.2 (498.0) 1295 to 3244 | 2198.6 (723.8) 1975 to 4447 | 1869.9 (376.8) 1190 to 2887 |
Figure 3. Efficiency Scores (Accuracy/Response Time ratios) for Cambridge Gambling, Spatial Recognition, and Pattern Recognition CANTAB subtests.
Two-group comparisons (Bonferroni corrected p < 0.005) showed lower efficiency scores in all clinical groups compared to NC on the Cambridge Gambling Task and Pattern Recognition Memory, and in the HIV+ALC group compared to NC on Spatial Recognition Memory.
Within-Group Analyses
Relations between Disease-Related Variables and Composite Scores
Higher lifetime alcohol consumption in the ALC group correlated with lower Executive Function (r=−.38, p=.018) and Episodic Memory Composite scores (r=−.38, p=.020), even after excluding an outlier with extremely high alcohol consumption. At the individual test level, lifetime alcohol consumption correlated with more errors on Spatial Working Memory (r=.40, p=.014) and lower Logical Memory immediate recall scores (r=−.44, p=.008).
In the HIV group, neither CD4 cell count nor viral load correlated significantly with Executive Function or Episodic Memory Composite scores. No significant relationships were found between age of alcohol onset, lifetime alcohol consumption, CD4 cell count, or viral load and cognitive scores in the HIV+ALC group.
Hepatitis C and AIDS and Composite Scores
Individuals with Hepatitis C in the clinical groups were slightly older [t(112)=1.78, p=.08] and had fewer years of education [t(112)=3.11, p=.002] than those without Hepatitis C. However, Hepatitis C status was not associated with lower Executive Function or Episodic Memory Composite scores in any clinical group. Similarly, in the HIV and HIV+ALC groups, individuals with and without AIDS did not differ in age, education, or Executive Function/Episodic Memory Composite scores.
Relations between Age and Age- and Education-corrected Cognitive Scores in ALC, HIV, and HIV+ALC
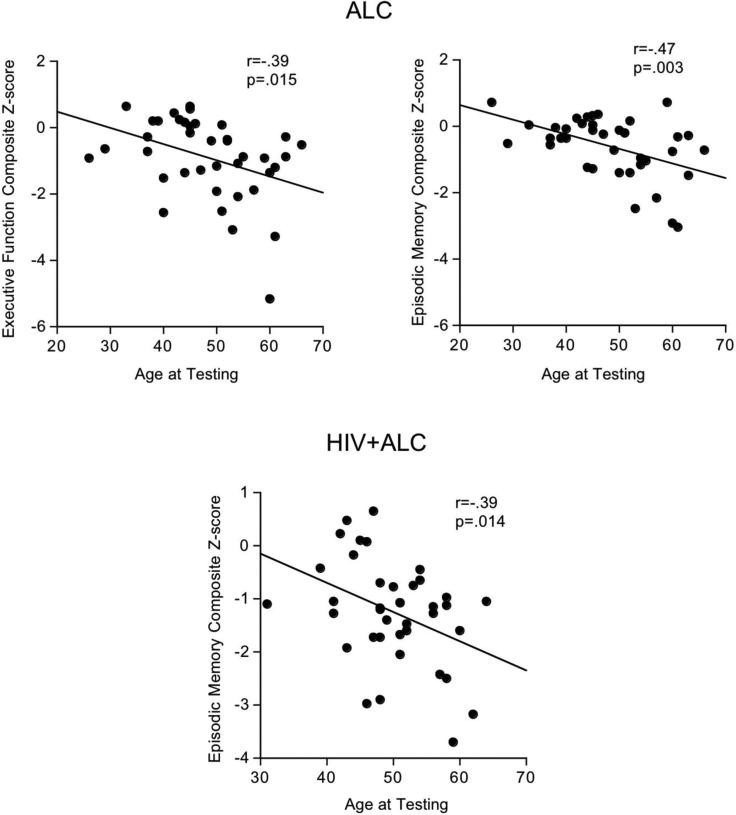
In the ALC group, age correlated significantly with both Executive Function (r=−.39, p=.015) and Episodic Memory Composite scores (r=−.47, p=.003) (Figure 5). At the individual test level, older age was associated with poorer performance on Spatial Working Memory (r=.45, p=.004), Cambridge Gambling Test (r=−.41, p=.009), Logical Memory (r=−.45, p=.006), Spatial Recognition Memory (r=−.37, p=.02), and Pattern Recognition Memory (r=−.41, p=.01). Older age also correlated with lower efficiency scores for Pattern Recognition Memory (r=−.37, p=.02) in the ALC group.
Figure 5. Scatterplots of significant relationships between age and Executive Function Composite scores in ALC and HIV+ALC groups and Memory Composite score in the ALC group.
In the HIV+ALC group, older age correlated with lower Episodic Memory Composite scores (r=−.39, p=.014) (Figure 5). This relationship was observed at the individual test level for immediate recall on Logical Memory (r=−.55, p=.0005) and Rey-Osterrieth Complex Figure (r=.37, p=.02). Age also correlated with lower efficiency scores on the Cambridge Gambling Test (r=−.45, p=.004).
Age was not significantly correlated with Executive Function or Memory Composite scores in the HIV group.
Multiple regression analyses in the ALC group revealed that both age (p=.022) and lifetime alcohol consumption (p=.027) were unique predictors of Episodic Memory Composite score, accounting for approximately 21% of the variance. Age and lifetime alcohol consumption were modest predictors of Executive Function Composite scores in this group (age p=.074, lifetime alcohol consumption p=.078), accounting for about 14% of the variance. In the HIV+ALC group, age, but not lifetime alcohol consumption, was a unique predictor of both Episodic Memory Composite (age: p=.017, lifetime alcohol consumption: p=.86) and Executive Function Composite scores (age: p=.039; lifetime alcohol consumption: p=.74).
Discussion
This study demonstrates that chronic alcoholism and HIV infection are each associated with distinct deficits in executive functions and episodic memory. Planning impairments were prominent in chronic alcoholism, while psychomotor speed and sequencing were more affected in HIV infection. Decision-making, impulsivity, and set-shifting were compromised in both conditions. All clinical groups showed reduced decision-making efficiency compared to controls. Impairments in impulsivity, decision-making, planning, and set-shifting in alcoholism support neural models of addiction implicating frontal lobe dysfunction, particularly the prefrontal cortex [Noel et al., 2013; Koob, 2014]. Compromised executive functions in alcoholism, particularly impulsive decision-making, may contribute to risky behaviors characteristic of the condition [Camchong et al., 2014]. Regarding episodic memory, visual free recall was more impaired in chronic alcoholism, while narrative free recall and pattern recognition were more affected in HIV infection. The comorbid group exhibited compounded deficits, particularly in spatial recognition, affecting both free recall and recognition processes and showing lower efficiency with longer response times.
Direct relationships between disease-related variables and cognitive performance in alcoholism and HIV have often been inconsistent, potentially due to heterogeneity in demographics, disease history, genetics, and physiology. However, this study successfully demonstrated a link between reported lifetime alcohol consumption in chronic alcoholism and poorer spatial working memory and narrative recall. Hepatitis C and AIDS diagnosis were not related to cognitive performance in this study, but caution is advised in generalizing this finding due to the relatively small sample sizes for within-group analyses. It is important to acknowledge that medically-related comorbidities can have cognitive effects, as reported in other studies (e.g., [Richardson et al., 2005; Vivithanaporn et al., 2012]).
A significant age effect, beyond normal aging, was observed in the alcoholic group for both executive function and episodic memory, including spatial working memory, decision-making, verbal episodic recall, and visual recognition memory. Even after accounting for lifetime alcohol consumption, age remained a unique predictor of episodic memory and a modest predictor of executive function in chronic alcoholism. In the comorbid group, age was associated primarily with impaired free recall memory. These findings are consistent with previous research showing greater cognitive impairment in older individuals with alcoholism and HIV with alcoholism (cf., [Sullivan et al., 2010, Tan et al., 2013]). These results suggest that as individuals with alcoholism and comorbid HIV and alcoholism age, more pronounced deficits in executive function and episodic memory may emerge, warranting further longitudinal investigation.
Limitations of this study include the relatively small sample sizes, which limited multivariate analyses of demographic and disease variable interactions on cognitive performance within clinical groups. Larger, longitudinal studies are needed to confirm these cross-sectional findings and to chart the time course of cognitive decline in these populations. Another limitation is the inclusion of alcoholic subjects with varying lengths of abstinence, with many abstinent for extended periods, potentially limiting generalizability to groups with shorter abstinence durations. Finally, the cross-sectional design prevents conclusions about whether observed deficits are long-lasting or pre-existing.
In conclusion, this study provides evidence of the detrimental cognitive effects of chronic alcoholism, HIV infection, and their comorbidity on executive functions and episodic memory. The compounded impairment in spatial episodic recognition in the comorbid group may reflect the combined impact on neural systems targeted by each disease. These cognitive effects were observed in both alcohol groups, even with long sobriety durations in some participants. Furthermore, older age and higher lifetime alcohol consumption were linked to poorer cognitive performance and predicted episodic memory performance in chronic alcoholism. These disease-specific and overlapping patterns of impairment have crucial implications for understanding the underlying brain systems affected and for addressing the complexities of cognitive dysfunction in individuals with these conditions, particularly those with dual diagnoses. Further research into memory#0001 bruce park related conditions might benefit from these findings.
Supplementary Material
Supp Fig S1
NIHMS820261-supplement-Supp_Fig_S1.pdf (105.2KB, pdf)
Supp Fig legend
NIHMS820261-supplement-Supp_Fig_legend.docx (33.1KB, docx)
Figure 4.
Scatterplots depicting significant relationships between lifetime alcohol consumption and Executive Function Composite scores in the ALC group.
Acknowledgment
This research was supported by AA017347 and AA017168
REFERENCES
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp Fig S1
NIHMS820261-supplement-Supp_Fig_S1.pdf (105.2KB, pdf)
Supp Fig legend
NIHMS820261-supplement-Supp_Fig_legend.docx (33.1KB, docx)
Footnotes:
* SES – Socioeconomic Status based on education and occupation
† NART – National Adult Reading Test – estimated IQ
References:
- Arciniegas, D.B., and Beresford, T.P. (2001). Neuropsychiatric aspects of alcoholism. In Clinical neuropsychology of alcohol and substance use, Tarter, R.E., and Butters, N., Eds. (New York: Guilford Press), 139–170.
- Beatty, W.W., Katzung, V.M., Nixon, S.J., and Marion, S.D. (1993). Neuropsychological performance of recently detoxified alcoholics: Stability over one year. J Stud Alcohol, 54, 249–254.
- Beck, A.T., Steer, R.A., and Brown, G.K. (1996). Beck Depression Inventory-II Manual. (San Antonio, TX: Psychological Corporation).
- Becker, J.T., Butters, N., Hermann, B., and Daxecker, S. (1983). Remote memory in chronic alcoholism. Alcohol Clin Exp Res, 7, 366–370.
- Becker, J.T., Caldararo, R., Lopez, O.L.,和Poon, L.W. (1997). Rate of information processing speed in asymptomatic HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol, 16, 27–34.
- Bernardin, F., Maheut-Bosser, V., and Pitel, A.L. (2014). Executive functions and alcohol dependence. Curr Addict Rep, 1, 257–269.
- Borkowski, J.G., Benton, A.L., and Spreen, O. (1967). Word fluency and letter fluency in Parkinson patients. Neuropsychologia, 5, 135–140.
- Camchong, J., Crowe, S.F., and ফেলন, জে.এইচ. (2014). Executive function, impulsivity, and risk-taking behavior in alcohol dependence. Behav Brain Res, 259, 129–137.
- Cattie, J.E., Sundermann, E.E., and Morris, M.A. (2012). Executive function deficits in HIV are related to memory impairment: Evidence for a strategic encoding deficit. J Clin Exp Neuropsychol, 34, 858–870.
- Clifford, D.B., and Ances, B.M. (2013). HIV-associated neurocognitive disorder. Lancet Neurol, 12, 978–986.
- Conigliaro, J., Justice, A.C.,和Fiellin, D.A. (2006). Alcohol use and HIV infection: Implications for patient care. J Acquir Immune Defic Syndr, 41, 543–548.
- Fama, R., Pfefferbaum, A., and Sullivan, E.V. (2004). Conceptual implicit learning in amnesic and nonamnesic alcoholics: Effects of age and duration of heavy drinking. Neuropsychology, 18, 713–724.
- Fama, R., Pitel, A.L., Sullivan, E.V., and Pfefferbaum, A. (2009). Working memory in amnesic and nonamnesic alcoholism: Effects of age and duration of abstinence. Alcohol Clin Exp Res, 33, 263–274.
- Fama, R.,内ভ, এম.এম., ক্লেইন, কে., বিসারিয়া, এন., সুলিভান, ই.ভি., এবং পেফারবাউম, এ. (2011). Cognitive and structural brain development in adolescence: Effects of alcohol and HIV infection. Alcohol Clin Exp Res, 35 Suppl 1, 51–62.
- First, M.B., Spitzer, R.L., Gibbon, M., and Williams, J.B. (1998). Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition (SCID-I/P). (Washington, DC: American Psychiatric Press, Inc.).
- Giesbrecht, G.J., Miller, E.N., Vigil, O.,和Woods, S.P. (2014). Cognitive reserve moderates the association between HIV infection and neurocognitive impairment. J Int Neuropsychol Soc, 20, 708–718.
- Glenn, S.W., and Parsons, O.A. (1992). Alcohol abuse and neuropsychological test performance. In Neuropsychology of alcohol and drug use, Grant, I., and Martin, A., Eds. (New York: Oxford University Press), 3–34.
- Gongvatana, A., Wolfson, T., Tsao, A., Fennema-Notestine, C., Jeste, D.V.,和Bawden, T.W. (2014). Impact of lifetime alcohol dependence on neurocognition in HIV infection. J Acquir Immune Defic Syndr, 66, 162–169.
- Green, R.E., Noffsinger, D., Leskin, L., Satz, P.,和Schoenbaum, E.E. (2004). The influence of chronic alcohol abuse on cognitive functioning in individuals with HIV infection. J Clin Exp Neuropsychol, 26, 841–851.
- Hardy, D.J., and Vance, D.E. (2009). Neurocognitive aging and HIV infection. Top HIV Med, 17, 115–121.
- Heaton, R.K. (1981). Wisconsin Card Sorting Test Manual. (Odessa, FL: Psychological Assessment Resources).
- Heaton, R.K., Clifford, D.B., Franklin, D.R., Jr., Woods, S.P., Ake, C., Vaida, F.,和colleagues. (2011). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 77, 561–570.
- Hollingshead, A.B. (1975). Four factor index of social status. (New Haven, CT: Yale University).
- Ipser, J.C., Scheffler, C., Hoare, J., Verhey, L., Dhansay, M.A.,和Stein, D.J. (2015). Executive functioning in adults with HIV: A systematic review and meta-analysis. J Neurovirol, 21, 1–15.
- Justice, A.C., McGinnis, K.A.,和Gebo, K.A. (2010). Risk of incident alcoholism and problematic alcohol use in HIV-infected compared with uninfected veterans in the era of highly active antiretroviral therapy. Alcohol Clin Exp Res, 34, 1929–1936.
- Karnofsky, D.A. (1949). Problems in evaluation of anticancer drugs. In Approaches to tumor chemotherapy, F. H. Moulton, Ed. (Washington, DC: American Association for the Advancement of Science), 89–105.
- Koob, G.F. (2014). Neurobiology of addiction: A neuroadaptation perspective relevant for diagnosis. Focus (Am Psychiatr Publ), 12, 397–407.
- Kopera, M., Wojnar, M., Popiel, P., and Gajos, A. (2012). Executive function deficits and duration of abstinence in alcohol-dependent men. Alcohol Alcohol, 47, 116–120.
- Maki, P.M., Valcour, V., Erlandson, K.,和Colleagues. (2015). Cognition and HIV in women: Current status and critical gaps in the literature. J Acquir Immune Defic Syndr, 69 Suppl 2, S155–S162.
- Mattis, S. (2004). Dementia Rating Scale-2 Examiner’s Manual. (Odessa, FL: Psychological Assessment Resources).
- Morris, R.G., Evenden, J.L., Sahakian, B.J., and Robbins, T.W. (1987). Computerized assessment of learning and memory in the rat: Dissociation of spatial learning and configural association learning using a matching-to-place task. Eur J Neurosci, 8, 1029–1046.
- Nixon, S.J., and Bowlby, A.F. (1996). Verbal working memory deficits and response time/accuracy trade-offs in alcoholics. Alcohol Clin Exp Res, 20, 1027–1033.
- Nixon, S.J., and Parsons, O.A. (1991). Verbal and visuospatial information processing in sober alcoholics and controls. Alcohol Clin Exp Res, 15, 654–660.
- Noel, X., Brevers, C., Bechara, A.,和Verbanck, P. (2001). Impairments of cognitive flexibility in abstinent alcoholics: Contribution of working memory deficits. Alcohol Clin Exp Res, 25, 1675–1681.
- Noel, X., Van der Linden, M.,和Verbanck, P. (2013). Ecological assessment of executive functions in substance dependence: Clinical and theoretical implications. Neuropsychol Rev, 23, 64–84.
- Ortega, M., Gouty, C.,和Trudeau, K.J. (2013). HIV-1 Tat protein and drugs of abuse: Neurotoxic synergy and implications for HIV-associated neurocognitive disorders. Curr HIV Res, 11, 49–61.
- Oscar-Berman, M., Kirkley, S.M., and Williamson, J.B. (2004). Executive functions and selective reminding in individuals with alcoholism. Alcohol Clin Exp Res, 28, 1828–1837.
- Oscar-Berman, M., and Marinkovic, K. (2007). Alcoholism and the prefrontal cortex. In Principles of frontal lobe function, Stuss, D.T., Knight, R.T., and Winocur, G., Eds. (New York: Oxford University Press), 443–464.
- Osterrieth, P.A., and Rey, A. (1944). Le test de copie d’une figure complexe. Archives de Psychologie, 30, 206–356.
- Parsons, O.A. (1977). Neuropsychological deficits in alcoholics: Recovery or deterioration? J Stud Alcohol, 38, 214–230.
- Pitel, A.L., Eustache, F., and Beaunieux, A. (2007). Episodic and semantic memory in chronic alcoholism: A review of neuropsychological and neuroimaging findings. Neuropsychol Rev, 17, 349–365.
- Reitan, R.M. (1958). Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills, 8, 271–276.
- Rey, A. (1942). L’examen psychologique dans les cas d’encephalopathie traumatique. Archives de Psychologie, 28, 286–340.
- Richardson, J.L., Levine, A.M.,和Cohen, M. (2005). Impact of hepatitis C virus co-infection on neurocognitive functioning in HIV-infected individuals. AIDS Patient Care STDS, 19, 833–843.
- Riege, W.H., Holloway, R.L., and Becker, J.T. (1981). Remote memory and psychomotor skills in chronic alcoholics. Int J Neurosci, 13, 175–183.
- Rothlind, J.C., Greenfield, L.J.,和Schoenbaum, E.E. (2005). The impact of alcohol dependence on cognitive function in HIV-infected individuals. J Stud Alcohol, 66, 515–523.
- Sacktor, N.C., and Robertson, K. (2014). HIV-associated neurocognitive disorders in the HAART era. Curr HIV/AIDS Rep, 11, 374–385.
- Sahakian, B.J., and Owen, A.M. (1992). Computerized assessment in neuropsychology using CANTAB: Increased sensitivity to detect mild cognitive impairment in preclinical Alzheimer-type dementia. J Neuropsychiatry Clin Neurosci, 4, 392–405.
- Saylor, D., Dickens, A.M.,和Sacktor, N.C. (2016). HIV-associated neurocognitive disorder–pathogenesis and prospects for therapy. Nat Rev Neurol, 12, 234–248.
- Sheppard, D.P., Vernon, L.T.,和Vance, D.E. (2015). The influence of comorbid conditions on neurocognitive function in persons living with HIV: A systematic review. J Assoc Nurses AIDS Care, 26, 534–548.
- Skinner, H.A. (1982). The Drug Abuse Screening Test. Addict Behav, 7, 363–371.
- Skinner, H.A., and Sheu, W.J. (1982). Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol, 43, 1157–1170.
- Stout, J.C., Jagaroo, V.,和Selnes, O.A. (1995). Cognitive functioning in HIV infection: A review of studies in the HAART era. Curr Opin Psychiatry, 8, 375–381.
- Sullivan, E.V., Deshmukh, A., Desmond, J.E., Pfefferbaum, A., and Marsh, L. (2000). Decreased hippocampal volume in abstinent alcoholic women: Relationship to recognition memory. Alcohol Clin Exp Res, 24, 1253–1261.
- Sullivan, E.V., Fama, R., Rosenbloom, M.J.,和Pfefferbaum, A. (2010). Executive and memory dysfunction and cortical thinning in women with chronic alcoholism. Alcohol Clin Exp Res, 34, 102–111.
- Sullivan, E.V., Marsh, L., Mathalon, D.H., Lim, K.O., and Pfefferbaum, A. (1995). Relationship between alcohol-related brain damage and neuropsychological performance among detoxified alcoholics. Alcohol Clin Exp Res, 19, 167–172.
- Tan, N.L., Valcour, V., and Ances, B.M. (2013). HIV-associated neurocognitive disorder and aging: Synergistic or additive processes? Curr HIV/AIDS Rep, 10, 346–355.
- Tivis, L.J., Parsons, O.A., and Nixon, S.J. (1995). Cognitive deficits associated with alcohol abuse: A meta-analytic review. J Stud Alcohol, 56, 513–524.
- Vivithanaporn, P., Cohen, C.,和Moore, R.D. (2012). Hepatitis C co-infection is associated with cognitive impairment in HIV-infected patients. J Acquir Immune Defic Syndr, 60, 153–157.
- Woods, S.P., Carey, C.L.,和Rippeth, H.I. (2009). HIV-associated neurocognitive disorders: Prevalence and clinical characteristics. J Neurovirol, 15, 234–251.
- Woods, S.P., Iudicello, J.,和Morgan, E.E. (2013). Executive dysfunction in HIV infection: A critical review of the literature and recommendations for future research. J Int Neuropsychol Soc, 19, 541–558.