1. Introduction
Event-related potential (ERP) research has consistently demonstrated the presence of atypical neural responses in infants at heightened risk for autism spectrum disorder (ASD) even before behavioral symptoms manifest [1,2,3,4,5,6]. Our prior work has specifically identified distinctive response patterns in the N290 and Negative central (Nc) ERP components to visual stimuli in two infant groups with elevated ASD risk: infant siblings of children with autism (ASIBs) and infants with fragile X syndrome (FXS) [4]. These groups face a significantly increased likelihood of developing ASD, with approximately 20% of ASIBs [7,8,9] and 60% of infants with FXS [10] receiving an ASD diagnosis, compared to 1.9% in the general population [11]. This study leverages cortical source analysis to gain deeper insights into the neural underpinnings of the N290 response in infants at high ASD risk.
The N290 component is intrinsically linked to face processing in infancy [12,13,14], and atypical face processing is a hallmark of visual attention differences in ASD [15,16,17]. Given that ASD diagnosis typically occurs in early childhood, investigating the N290 and its cortical origins during infancy is crucial for understanding the development of face processing in autism and for identifying early markers of ASD risk. Cortical source analysis, which combines scalp-recorded electrophysiological data with a head model, offers a non-invasive and infant-friendly method to estimate neural sources within the brain. This study marks the first exploration of neural generators of ERP responses, including the N290, in infants at elevated ASD risk.
1.1. The Infant N290 Component
Examining the infant N290 ERP component holds particular significance for understanding ASD development. The amplitude of the N290 has been correlated with the manifestation of ASD symptoms in later childhood [18]. Notably, Shephard and colleagues [18] found that greater N290 amplitudes to faces versus noise at 7 months old in ASIBs, but not in typically developing (TD) infants, were associated with more pronounced social-communication impairments at 7 years of age. The N290 is considered a reflection of developing face specialization [12,13] and is believed to be a precursor to the adult N170 component [14,19,20,21]. Characteristically, the N290 presents as a negative peak around 290 ms post-stimulus onset at lateral posterior electrode sites [12,13]. It exhibits a greater amplitude in response to faces compared to other stimulus categories [12,13,14].
Discrimination between face and non-face stimuli at the N290 level has been observed in studies involving ASIBs [4,6] and infants with FXS [4]. Guy et al. [4] investigated ERP responses in 12-month-old ASIBs, infants with FXS, and low-risk control (LRC) infants to images of the participant’s mother’s face, a stranger’s face, the participant’s favorite toy, and a novel toy. Infants with FXS showed an amplified N290 response to their mother’s face and a reduced response to the novel toy, leading to significantly greater N290 amplitude for familiar versus novel stimuli. This unique pattern of N290 responses based on stimulus familiarity was exclusive to infants with FXS, whose responses were also stronger than those of ASIB and LRC infants to both familiar and novel stimuli. Key and Stone [22], and Luyster and colleagues [23] investigated responses to mother’s and stranger’s faces in ASIBs and LRC infants. Key and Stone [22] found a larger amplitude N290 response to the mother’s face compared to a stranger’s face in both 9-month-old LRC infants and ASIBs. However, Luyster et al. [23] reported only a marginally significant difference in N290 amplitude for LRC infants, with a more negative N290 for stranger’s faces. ASIBs in their study did not show face discrimination based on N290 amplitude [23]. These findings highlight variability potentially influenced by stimuli, age, and ERP processing methods. While most studies include both face and non-face stimuli, some investigating familiarity effects on N290 amplitude have focused solely on familiar and novel faces [22,23]. Further research is necessary to fully elucidate the role of familiarity in the infant N290.
1.2. Cortical Source Analysis of the N290
Cortical source analysis is instrumental in deciphering the neural mechanisms behind the N290 differences observed at the scalp level between infants with FXS and ASIBs. It’s hypothesized that the enhanced amplitude responses in FXS might reflect synaptic hyperresponsivity [27], whereas the muted responses in ASIBs could indicate hyporesponsivity. Investigations into the sources of the N290 have been conducted in typically developing infants [12,13,28,29]. Recent studies on developing face specialization in infants aged 3 to 12 months have identified the fusiform gyrus as the generator of the N290 [12,13]. The middle fusiform gyrus exhibited greater activation for faces compared to toys, with a peak activation time coinciding with the N290 peak. While other areas like the anterior fusiform gyrus and parahippocampal gyrus also showed increased activity to faces during the N290 time window, they didn’t exhibit peak activation at the N290 peak latency. It is important to note that increased activation alone does not confirm an ROI as the source of a component. The parahippocampal gyrus, for instance, shows a linear increase in activation during the N290 window and might contribute to the later P400 component.
The N290 is considered a developmental precursor to the adult N170 ERP component [12,13,14], and findings are consistent with cortical source analysis results for the N170. The N170 is strongly associated with face processing in adults, showing larger amplitudes for faces versus non-face stimuli like houses and objects [30,31,32,33,34,35,36]. While multiple sources contribute to the N170 (summarized in 37 supplemental information), the fusiform gyrus, particularly the middle and posterior sections, is most consistently identified as the primary source [37,38,39,40]. The fusiform gyrus’s role in face processing is further supported by research combining ERP and fMRI measurements in adults [37,41,42,43]. Interestingly, decreased fusiform gyrus activation has been reported in individuals with FXS [44], suggesting that the enhanced activation during face processing in infants with FXS may not originate from typical N290/N170 sources.
1.3. Head Model Selection for N290 Source Analysis
Utilizing realistic head models derived from individual participants’ own MRIs is the gold standard for electrical source analysis [12,13,45]. However, obtaining MRIs for every participant is often impractical due to cost and compliance issues. A viable alternative is employing head models based on average MRI templates. Many EEG/ERP source analysis methods use templates from the Montreal Neurological Institute (MNI) [46,47]. Age-specific MRI templates for infants or children are preferable to adult templates [48], as reviewed in [49]. Rapid brain development in early infancy leads to significant variability in brain size, shape, and tissue, even within narrow age ranges [50,51,52,53,54,55]. Recent advancements have made age-specific MRI templates available across the lifespan [53,54,55,56,57,58].
Considering known neural and neurophysiological differences across participant subgroups is crucial. Infants at high risk for ASD exhibit atypical brain development patterns. Studies indicate that children later diagnosed with ASD show larger than average head circumference by 12 to 24 months, potentially reflecting brain overgrowth [59,60,61,62]. Cortical surface hyperexpansion between 6 and 12 months has been observed in ASIBs who later showed brain overgrowth and were diagnosed with ASD [63]. Moreover, both children with idiopathic ASD and FXS show increased brain volume compared to typically developing children, with different enlargement patterns across groups [64]. These structural differences, potentially present in infancy, support the use of distinct head models for ASIBs and infants with FXS to enhance the accuracy of source analysis.
1.4. Objectives of the Current N290 Study
This study had two primary objectives. First, we aimed to evaluate alternative head models for use with etiologically distinct infants at high ASD risk (ASIBs and FXS). Realistic head models from individual MRIs are considered optimal for cortical source analysis [65,66]. We assessed the similarity of head models derived from infant-specific MRIs with those based on average MRI templates. These templates included study-specific averages for both risk groups (SS-ASIB, SS-FXS), group-specific averages from the Infant Brain Imaging Study (IBIS) database (IBIS-ASIB, IBIS-FXS), a template from 12-month-old typically developing infants (12-mo-TD), a 12-year-old TD child template (12-yr-TD), and a TD adult template (Adult-TD). These alternatives were chosen to represent common research practices: using models from similar risk groups (SS, IBIS), same-age infants (12-mo-TD), and readily accessible models (12-yr-TD, Adult-TD). Comparing results based on individual MRIs versus average templates will guide the selection of appropriate average MRI templates for future N290 research.
Second, we investigated brain regions associated with face-sensitive ERP responses in high-risk infants using cortical source analysis and a pediatric-validated pipeline [48]. The head model comparison results informed this goal. Individual MRI-derived head models were used when available, and the best average template substitute was used otherwise. We analyzed N290 data from a previous study [4] to determine if scalp activation differences to social stimuli across risk groups are due to variations in brain activation areas or activation amplitude. Focusing on the N290 ERP component and its known cortical sources [12,13,37], we compared the cortical source of the N290 in 12-month-old ASIB, FXS, and LRC infants. This research aims to improve our understanding of the neural sources underlying the distinct ERP activity patterns observed in these high-risk infant groups, particularly in relation to the N290 response.
2. Materials and Methods
2.1. Participants
Fifty-seven 12-month-old infants participated, including 21 ASIBs (18 males), 15 infants with FXS (8 males), and 21 typically developing LRC infants (16 males). For the head model comparison, only high-risk infants with structural MRI recordings (8 ASIBs, 11 FXS) were included. All participants were included in the cortical source analysis of the N290 data.
2.2. Procedure
2.2.1. EEG Recording
Infants were fitted with an EGI Hydrocel Geodesic Sensor Net at the University of South Carolina Infant Development Lab. Seated on a parent’s lap facing a monitor, they viewed stimuli. A video camera recorded looking behavior. Stimulus presentation was controlled remotely. ERP stimuli (500 ms duration) included photos of the participant’s mother, a stranger’s mother, the participant’s toy, and a novel toy. Sesame Street clips were used to maintain attention. Inter-trial intervals varied from 500–1500 ms. See Guy et al. [4] for further EEG recording details relevant to the N290 component.
2.2.2. Magnetic Resonance Imaging
Infant MRI data were collected within 30 days of their 12-month birthday using a Siemens 3T Trio scanner at Palmetto Health Hospital in Columbia, SC, during natural sleep (no sedation). Infants were prepared with earplugs and headphones and positioned in the scanner with foam padding. An MRI technician, parent, and nurse were present during the ~15-minute scan, which included localizer, 3D sagittal T1-weighted, and T2-weighted sequences, covering from the top of the head to the neck.
An additional 77 structural MRIs were obtained from the Infant Brain Imaging Study (IBIS): 53 from 12-month-old ASIBs (27 later diagnosed with ASD, 26 not diagnosed) and 24 from 12-month-old infants with FXS (ASD outcomes not available). All IBIS scans were used for IBIS-ASIB and IBIS-FXS head model creation. IBIS participants were separate from the ERP/MRI study participants.
2.3. Data Processing
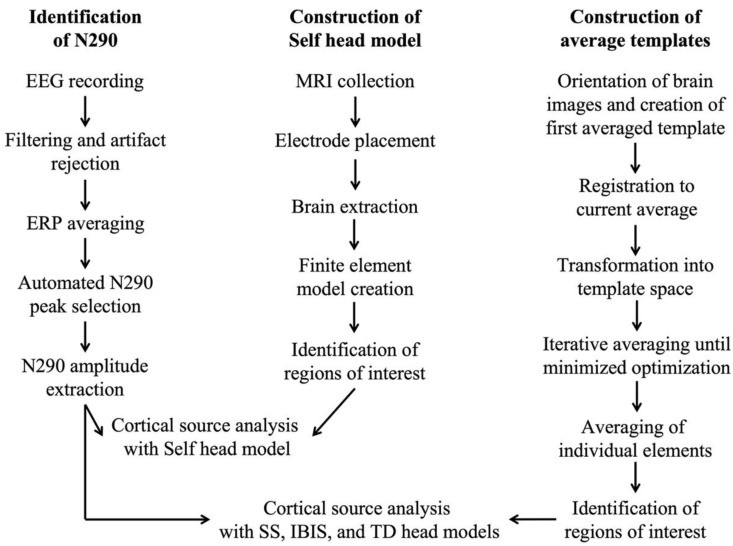
Figure 1 illustrates the EEG and MRI data processing workflows. Imaging data was processed at individual (Self head models) and group levels (average templates).
Figure 1.
Data processing workflow for EEG, ERP, and MRI data, showing “Self head model” construction for cortical source analysis with individual MRIs and “average template” construction for IBIS, SS, and TD averaged head models.
2.3.1. MRI File Preparation
MRI files were processed following Richards et al. [53,54,56]. Brains were extracted using FSL brain extraction tools, visually inspected, and adjusted using bet2 variables for accuracy [67].
Realistic head models were created from structural MRIs by segmenting and assigning conductivity values to head tissues: gray matter, white matter, non-neural brain tissue, cerebrospinal fluid, skull, scalp, eyes, nasal cavity, and extracranial areas. Finite element method (FEM) models were created for each MRI, considering voxel-level geometry and conductivity. FEM model validity with tissue segmentation is empirically supported [68]. Conductivity values are in Table 1: 0.35 S/m scalp, 0.0132 S/m skull, 1.79 S/m cerebrospinal fluid, 0.2 S/m white matter, 0.33 S/m gray matter, 0.33 S/m dura, 0.35 S/m muscles, 0.5 S/m eyes, and 0.0048 S/m nasal cavity.
Table 1.
Conductivity values of head model segments.
| Segment | Conductivity (S/m) |
|---|---|
| Scalp | 0.3500 |
| Skull | 0.0132 |
| Cerebrospinal fluid | 1.7900 |
| White matter | 0.2000 |
| Gray matter | 0.3300 |
| Dura | 0.3300 |
| Muscles | 0.3500 |
| Eyes | 0.5000 |
| Nasal cavity | 0.0048 |
Note. S/m = Siemens per meter.
2.3.2. Construction of Group- and Study-Specific Templates
Average head models (study-specific: SS-ASIB, SS-FXS; IBIS-ASIB, IBIS-FXS) were created using iterative procedures [53,54,56]. Templates were made for both whole head and extracted brain. First, preliminary averages were created using rigid rotation (FLIRT) [69] to a 12-month-old TD template [53]. Second, non-linear registration (ANTS) [70,71], transformation, and averaging were iterated until root mean square difference stabilized. See Sanchez et al. [53,54] for details.
Comparison head models were based on average MRI templates from the “Neurodevelopmental MRI Database” [58]: 12-month-old TD infants (12-mo-TD), 12-year-old TD children (12-yr-TD), and 20–24-year-old TD adults (Adult-TD). These templates are updated and include MRIs from 169 12-month-olds, 139 12-year-olds, and 632 adults.
2.3.3. Electrode Placement
EGI Hydrocel 128-electrode locations were computed on average MRI templates. For average template models [58], 12-month-old infant electrode locations were estimated from photos of EEG nets on participants, matching fiducial electrodes to fiducials on average MRI templates. Remaining locations were estimated based on net connections. 12-year-old and adult template electrodes were derived from Geodesic Photogrammetry System measurements. Electrode placements were co-registered to MRIs, averaged, and placed on average MRI templates.
Study participant electrode placements were created like the 12-month average template. Study-specific template (SS-ASIB, SS-FXS) placements were averaged from group subjects and fitted to templates. IBIS group template electrode placements were derived by fitting study-specific template placements onto group templates.
2.3.4. Regions of Interest
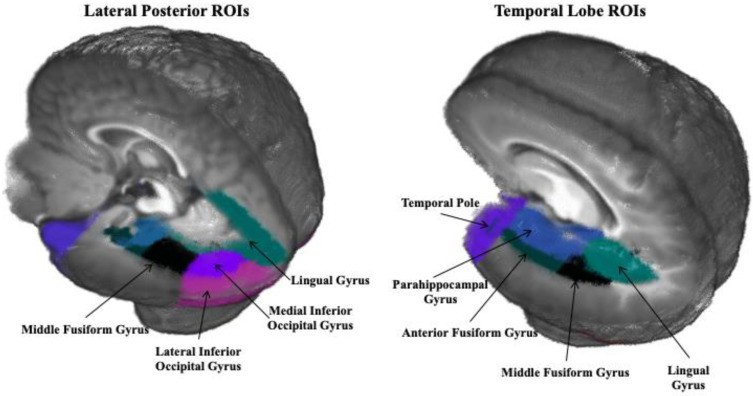
Regions of interest (ROIs) were selected based on prior N290 source studies [12,13], including dorsal anterior cingulate gyrus, frontal pole, anterior fusiform gyrus, middle fusiform gyrus, lateral inferior occipital gyrus, medial inferior occipital gyrus, lingual gyrus, middle occipital lobe, middle temporal gyrus, orbital frontal gyrus, parahippocampal gyrus, posterior cingulate gyrus, superior occipital lobe, superior parietal lobe, superior temporal gyrus, superior temporal sulcus, temporal pole, and ventral anterior cingulate. Figure 2 shows ROIs near N290 sources and control areas.
Figure 2.
Regions of interest (ROIs) on a 6-month-old average template (Guy, Zieber, and Richards, 2016 [13]).
2.4. Data Analysis
2.4.1. Head Model Comparison
Source analysis results from Self head models were compared to: (a) SS-ASIB/FXS; (b) IBIS-ASIB/FXS; (c) 12-mo-TD; (d) 12-yr-TD; and (e) Adult-TD head models. ANOVAs examined activation differences across ROIs and head models. Pearson correlations assessed activation pattern relatedness. Current density reconstruction (CDR) from ROIs were dependent variables, correlated between participant MRI and average template head models. Strong positive correlations indicated high similarity.
2.4.2. Cortical Source Analysis of N290
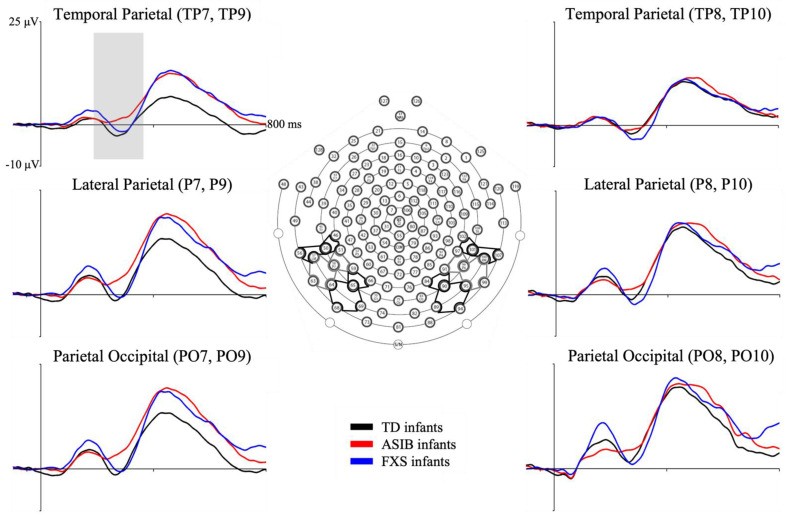
Cortical source analysis of N290 ERP data used Self head models (when available) and the best substitute (otherwise). N290 peaks were individually identified for each stimulus and participant at parietal-occipital (PO7, PO8, PO9, PO10), parietal (P7, P8, P9, P10), and temporal-parietal (TP7, TP8, TP9, TP10) electrodes (Figure 3).
Figure 3.
Grand average N290 responses by group at PO, P, and TP electrode clusters. Inferior posterior electrode clusters of interest on EGI Hydrocel layout. (From Guy, Richards, Tonnsen, and Roberts (2018) [4]). Note. TD = typical development, ASIB = infant sibling of child with autism, FXS = fragile X syndrome.
A MATLAB script identified the N290 peak amplitude between the P1 peak and 400 ms [72]. Peaks were inspected and adjusted. If peaks were undetectable, activity at 290 ms was used. Peak amplitude N290 was change from P1 to N290 peak. Fieldtrip toolbox in MATLAB estimated cortical sources with current density reconstruction (CDR) [73] using eLORETA [74]. CDR was calculated for 20 ms around individual N290 peaks. CDR results show current amplitude per voxel within the head model during the N290 window. CDR was summed over each ROI voxel and divided by ROI volume for average current per mm3.
3. Results
3.1. N290 ERP Component
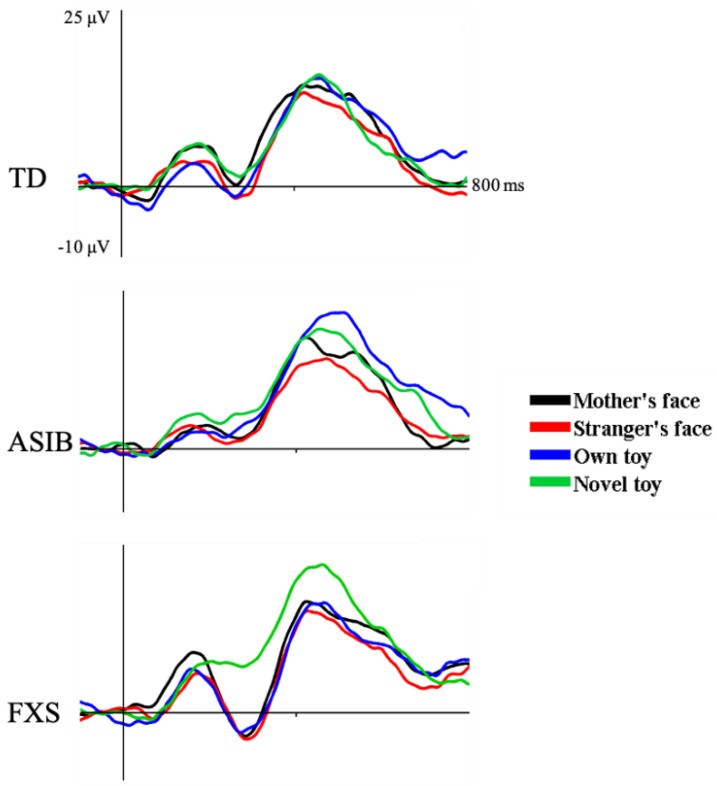
The N290 appeared as a negative peak around 300 ms post-stimulus (Figure 3). Figure 4 shows N290 responses per stimulus type, averaged across electrodes, per group. A significant main effect of stimulus type was found: N290 responses to faces were greater than to toys. The interaction between group and stimulus type was not significant, although the face-toy difference was largest in FXS infants. A marginally significant group by familiarity interaction occurred due to significantly greater N290 amplitude to familiar stimuli in FXS infants compared to ASIB and LRC groups and all groups’ responses to novel stimuli. Only FXS infants differentiated stimuli by familiarity (see [4] for details).
Figure 4.
N290 responses to mother’s face, stranger’s face, own toy, and novel toys for each participant group.
3.2. Head Model Comparison
CDR activation from Self head models was compared to IBIS, study-specific, 12-Month TD, 12-Year TD, and Adult TD models. A Group (ASIB, FXS) × Head Model × ROI ANOVA was conducted.
Table 2 shows CDR activation to faces and toys by head model. CDR activation was most similar between Self and IBIS head models. A main effect of head model was significant, F(5, 85) = 15.95, p < 0.001, and ROI, F(17, 289) = 5.05, p < 0.001.
Table 2.
Mean CDR amplitude and CDR amplitude in response to faces and toys across ROIs for Self head model and other models. Negative difference scores indicate lower mean CDR amplitudes than Self.
| CDR per mm3 | |||
|---|---|---|---|
| Head Model | Description | Mean | Faces |
| Self | Created from participant’s own MRI | 3.39 | 3.97 |
| IBIS-ASIB/IBIS-FXS | Created from 53 (ASIBs) or 24 (FXS) IBIS MRIs | −0.05 | −0.08 |
| SS-ASIB/SS-FXS | Created from 8 (ASIBs) or 11 (FXS) study MRIs | −1.02 | −0.63 |
| 12-mo-TD | Created from 169 Neurodevelopmental MRI Database MRIs | −0.59 | −0.76 |
| 12-yr-TD | Created from 139 Neurodevelopmental MRI Database MRIs | −0.91 | −1.12 |
| Adult-TD | Created from 632 Neurodevelopmental MRI Database MRIs | −0.60 | −0.77 |
Note. ASIB = infant siblings of children with autism; CDR = current density reconstruction; FXS = fragile X syndrome; IBIS = infant brain imaging study; MRI = magnetic resonance image; SS = study-specific; TD = typically developing.
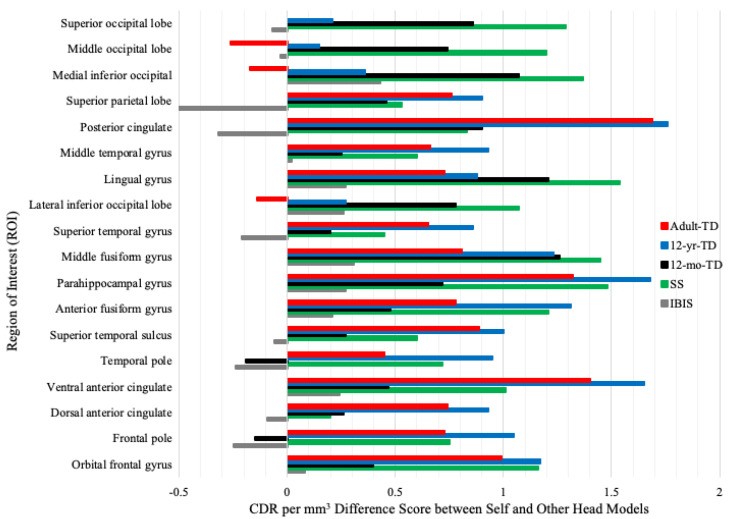
Figure 5 shows CDR difference scores (Self minus other models) across ROIs. IBIS head models had lower difference scores than others. For face processing ROIs (middle, anterior fusiform gyri, parahippocampal gyrus), only IBIS models had difference scores within 0.5 CDR per mm3 of Self. A head model by ROI interaction was significant, F(85, 1445) = 12.41, p < 0.001 (Figure 5). A group by head model by ROI interaction was also significant, F(85, 1445) = 1.48, p = 0.004. Self and IBIS models fit best for ASIBs and FXS infants, but fit for other models varied by group. Table 3 shows CDR data for Self, SS, and IBIS models at each ROI, sorted by activation, for ASIBs and FXS infants. Face processing ROIs are highlighted yellow; SS or IBIS CDRs closest to Self are green. IBIS models more closely reflected Self models for both groups, with some ROI variability in ASIBs.
Figure 5.
Difference scores (Self CDR – other models CDR) at each ROI.
Table 3.
Mean CDR amplitude for ASIBs and FXS infants at each ROI for Self, SS, and IBIS head models. Yellow highlights indicate N290 source ROIs in TD infants. Green highlights indicate SS or IBIS model closest to Self at each ROI.
| ASIB | FXS |
|---|---|
| Head Model | Head Model |
| ROI | Self |
| Parahippocampal gyrus | 4.65 |
| Anterior fusiform | 4.42 |
| Lingual gyrus | 4.35 |
| Middle fusiform | 4.08 |
| Posterior cingulate | 3.78 |
| Medial inferior occipital | 3.63 |
| Superior occipital lobe | 3.53 |
| Temporal pole | 3.50 |
| Orbital frontal gyrus | 3.26 |
| Superior parietal lobe | 3.20 |
| Lateral inferior occipital | 3.05 |
| Middle occipital lobe | 3.05 |
| Ventral anterior cingulate | 3.03 |
| Middle temporal gyrus | 2.65 |
| Superior temporal sulcus | 2.53 |
| Superior temporal gyrus | 2.53 |
| Frontal pole | 3.50 |
| Dorsal anterior cingulate | 3.03 |
Note. CDR = current density reconstruction; ROI = regions of interest; IBIS = Infant Brain Imaging Study; SS = study-specific.
Pearson correlations (Table 4) of CDR activation between Self and other models (IBIS, SS, 12-mo-TD, 12-Yr-TD, Adult-TD) across ROIs, for faces and toys, showed IBIS models best correlated with Self models. ASIBs had very high Self-IBIS correlation, FXS infants showed less strong and consistent correlations.
Table 4.
Pearson correlations between CDR activation using Self head model and average-template-derived head models.
| Correlation with Self Head Model (SD) | |||
|---|---|---|---|
| Head Model | Overall | ASIB | FXS |
| IBIS-ASIB/IBIS-FXS | .84 (.12) | .88 (.07) | .81 (.14) |
| SS-ASIB/SS-FXS | .81 (.13) | .81 (.17) | .81 (.10) |
| 12-mo-TD | .81 (.15) | .78 (.18) | .82 (.12) |
| 12-yr-TD | .70 (.12) | .77 (.12) | .66 (.26) |
| Adult-TD | .74 (.18) | .78 (.12) | .72 (.22) |
Note. CDR = current density reconstruction; IBIS = Infant Brain Imaging Study; SS = study-specific; TD = typically developing; SD = standard deviation.
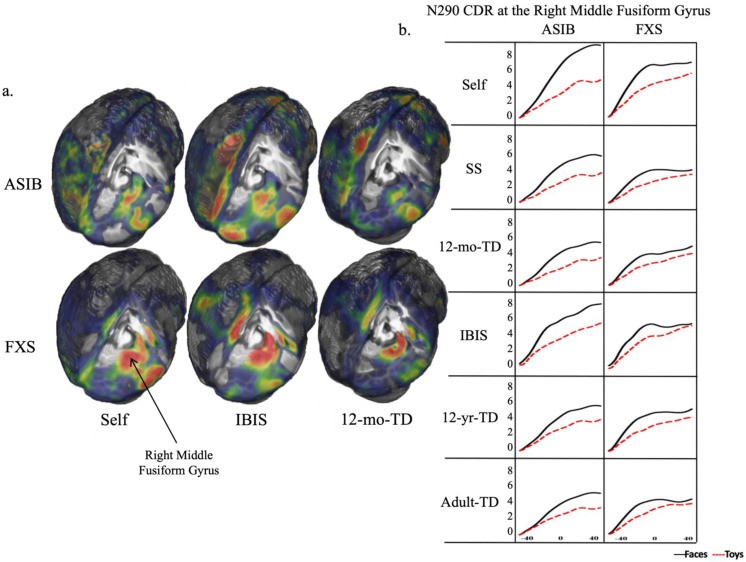
IBIS group-specific head models were the best Self model substitute. Figure 6 shows CDR activation patterns on whole head and right middle fusiform gyrus for each model. IBIS models yielded activation patterns closest to Self models in amplitude and peakedness. ASIBs showed increasing right middle fusiform gyrus activation over time, seen across models, but only IBIS models approached Self model activation levels. FXS infants showed activation peaking with N290, then stabilizing, best matched by IBIS models, in both whole-head and middle fusiform gyrus plots.
Figure 6.
(a). CDR activation areas and degree on Self, IBIS, and 12-mo-TD whole-head models for ASIBs and FXS infants; warmer colors = greater activation; (b). CDR activation patterns at right middle fusiform gyrus during N290 window for each head model and group.
3.3. Cortical Source Analysis of N290
Cortical source analysis of N290 was conducted for all EEG participants using individual head models (if MRI available) or IBIS head models (otherwise), based on head model comparison results.
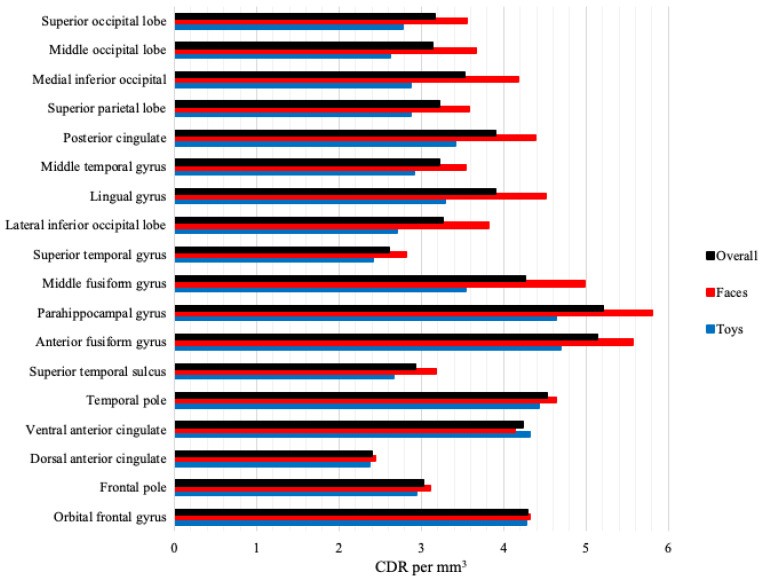
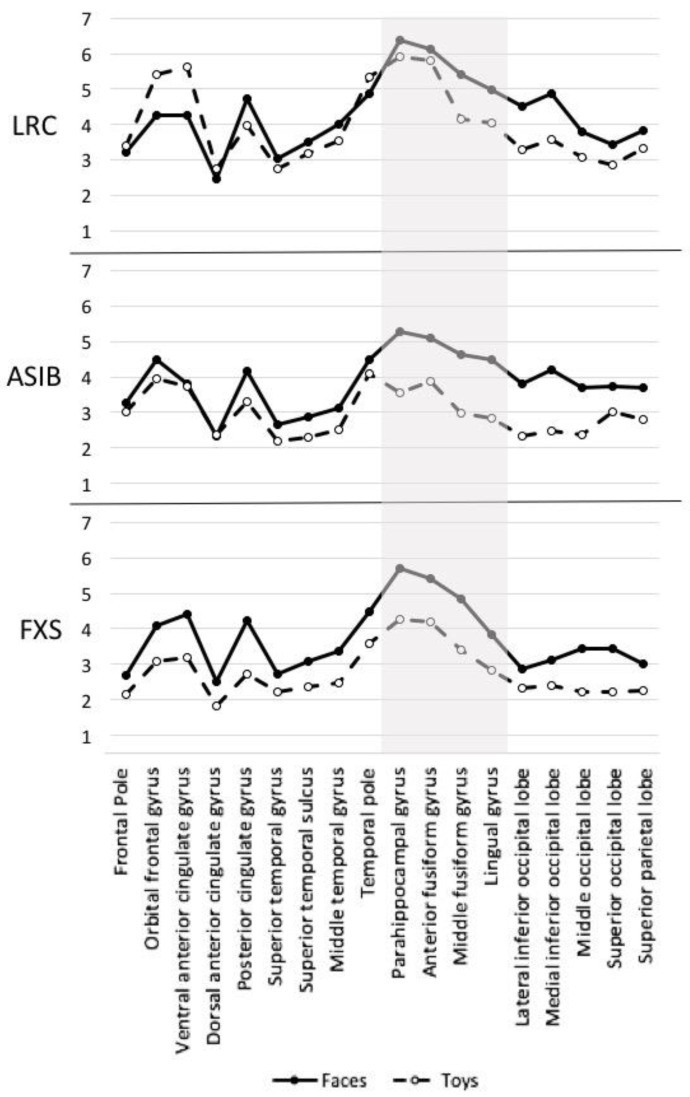
Source analysis CDR values were analyzed by stimulus type, group, and ROI. Figure 7 shows overall CDR activation and face/toy CDR at each ROI across groups. Activation was highest in anterior fusiform and parahippocampal gyri, but face-toy differentiation was greatest in middle fusiform gyrus. These effects were significant. A Group × Stimulus Type × ROI ANOVA showed significant main effects of stimulus type, F(1, 55) = 21.47, p < 0.001, faces (M = 4.08) > toys (M = 3.31), and ROI, F(17, 935) = 27.67, p < 0.001, highest in anterior fusiform (M = 5.13) and parahippocampal gyri (M = 5.21). A stimulus type by ROI interaction was significant, F(17, 935) = 4.91, p < 0.001; face-toy difference largest in middle fusiform gyrus (faces M = 4.98, toys M = 3.53). A significant group by stimulus type by ROI interaction, F(34, 935) = 1.45, p = 0.047, was also found. Figure 8 shows face/toy CDR activation per group and ROI. FXS infants showed greater face > toy activation across all ROIs; ASIBs showed this at all but dorsal anterior cingulate. LRC infants showed specialization, with face > toy activation in most, but not all, ROIs.
Figure 7.
CDR activation levels across ROIs for cortical source analysis.
Figure 8.
CDR activation to faces and toys for each group and ROI; N290 source ROIs highlighted.
4. Discussion
4.1. Head Model Comparison
This study assessed head model substitutes based on activation level and pattern similarity, aiming to determine if specific head models are needed for each high-risk group’s source analysis, particularly in relation to the N290. We hypothesized that group-specific realistic head models would be most accurate compared to Self models, and TD 12-year-old/adult models would be poor substitutes for N290 analysis.
Comparing five head models (SS, IBIS, 12-mo-TD, 12-yr-TD, Adult-TD) to Self models, IBIS group-specific models consistently emerged as the best substitutes. Tables 2, 3, 4 and Figures 5, 6 demonstrate IBIS models yielded activation patterns closest to Self models, particularly for the N290 component. Figure 6 shows IBIS-FXS models best captured the activation peak in the right middle fusiform gyrus synchronous with the N290 peak in FXS infants. Similarly, IBIS-ASIB models best matched Self model activation levels in ASIBs for the N290.
The superior performance of IBIS models over study-specific models is notable. A larger MRI collection in IBIS models may capture greater heterogeneity, especially in ASIBs, a highly diverse group. Atypical brain development in high-risk infants, including brain overgrowth in those later diagnosed with ASD [59,60,61,62,63], further supports the need for group-specific templates for accurate source analysis, especially for the N290 component. Brain overgrowth sites include middle occipital, right cuneus, right lingual, left inferior temporal, and middle frontal gyri [63].
While IBIS models were best for FXS infants, the effects were less robust. FXS infants showed similar correlations between Self, IBIS, SS, and 12-month TD infant models (Table 3). Potentially, ROIs examined were less heterogeneous in FXS infants. Structural abnormalities in FXS infants and young children are documented [75,76,77].
The importance of neuroanatomically specific head models aligns with findings that large-scale ASD brain structure studies can be misleading, and homogenous subgroups are needed for accurate results [78,79,80]. Chen et al. [78] demonstrated improved accuracy by subgrouping participants based on neuroanatomical differences. This study did not include ASD outcomes or clinical characteristics. Self head models are recommended when available. However, group-specific head models from same-age participants are a valid substitute when Self models are not accessible for N290 analysis.
Limitations include the need to empirically verify why IBIS models outperform study-specific models. Investigating head model reliability with varying numbers of scans in homogenous/heterogeneous groups would be beneficial for future N290 research.
4.2. Cortical Source Analysis of N290
This first cortical source analysis of ERP activity in infants at high autism risk provides evidence for distinct neural activation patterns during face processing across risk groups, particularly related to the N290. All infants showed greater face vs. toy activation in face processing areas like middle fusiform gyrus, anterior fusiform gyrus, parahippocampal gyrus, and lingual gyrus, mirroring TD infant N290 source analysis [12,13]. Widespread, high face activation in FXS infants likely contributes to their enhanced N290 amplitude. These findings suggest that greater N290 amplitude in FXS infants stems from increased activation area during face processing, not just increased activation in specialized face processing regions.
This study advances understanding of the N290 in high-risk infants. Prior work showed distinct N290 activation patterns in ASIBs and FXS infants [4], possibly reflecting varied pathways to social-communication impairments in ASD. FXS infants showed hyper-reactivity based on enhanced N290 responses, while ASIBs showed hypo-reactivity with muted ERP responses. Shephard et al. [18] linked greater stimulus-type discrimination in infant N290 to atypical N170 lateralization and more severe social-communication impairment in ASIBs at age 7. Different neural sources may contribute to enhanced N290 associated with emerging ASD symptoms across groups. Future research should explore infant neural responses and outcome data. Longitudinal follow-up of participants shows ASD diagnoses in 19% of ASIBs and 33% of FXS infants, but group sizes are currently too small for statistical analysis. Future datasets should be supplemented. New head models should be created with diagnosis in mind (ASD vs. non-ASD ASIBs) to better understand cortical sources in relation to outcome data for N290 research.
Resulting head models can aid in early ASD identification. Templates from this study (T1W/T2W head/brain, age-specific) are available to researchers via nitrc.org (http://jerlab.psych.sc.edu/neurodevelopmentalmridatabase, accessed 12 July 2022).
5. Conclusions
This study underscores the importance of appropriate head models for accurate cortical source analysis, especially in N290 research. Head model comparisons for infant ASIB and FXS ERP data indicate unique participant groups require unique head models. Etiologically distinct high-risk infant groups differ in brain structure and function, crucial for head model selection. N290 cortical source analysis revealed developing face specialization across all groups, shown by greater face vs. toy activation in face processing ROIs like middle fusiform gyrus. However, subtle activation differences exist across high-risk groups: increased face activation across ROIs in FXS infants and muted ROI activation in ASIBs for the N290 component. Continued research is vital to enhance understanding of cortical development in social information processing in neurodevelopmental disorders like FXS and ASD, particularly in relation to the N290 response.
Acknowledgments
The authors thank Heather Hazlett and the Infant Brain Imaging Study (IBIS) team for sharing structural MRIs.
Abbreviations
| ASD | autism spectrum disorder |
|---|---|
| CDR | current density reconstruction |
| ERP | event-related potential |
| FEM | finite element model |
| FXS | fragile X syndrome |
| IBIS | Infant Brain Imaging Study |
| ASIB | infant siblings of children with autism |
| LRC | low-risk control |
| MNI | Montreal Neurological Institute |
| Nc | Negative central |
| ROI | region of interest |
| SS | study-specific |
| TD | typically developing |
Author Contributions
Conceptualization, M.W.G. and J.E.R. (John E. Richards); methodology, M.W.G. and J.E.R. (John E. Richards); formal analysis, M.W.G.; resources, J.E.R. (John E. Richards) and J.E.R. (Jane E. Roberts); writing—original draft preparation, M.W.G.; writing—review and editing, M.W.G., J.E.R. (John E. Richards) and J.E.R. (Jane E. Roberts); visualization, M.W.G. and J.E.R. (John E. Richards); funding acquisition, J.E.R. (John E. Richards) and J.E.R. (Jane E. Roberts). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The Institutional Review Board of the University of South Carolina approved this study.
Informed Consent Statement
Parents/guardians provided written consent.
Data Availability Statement
Templates are available at nitrc.org (http://jerlab.psych.sc.edu/neurodevelopmentalmridatabase) and ERP data at NDAR (https://nda.nih.gov). Contact John E. Richards ([email protected]).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by grants R37 HD018942 and NIMH-R01MH090194.
Footnotes
Publisher’s Note: MDPI stays neutral.
References
Associated Data
Data Availability Statement
Templates are available at nitrc.org (http://jerlab.psych.sc.edu/neurodevelopmentalmridatabase) and ERP data at NDAR (https://nda.nih.gov). Contact John E. Richards ([email protected]).
